Xenon Tetrafluoride
Intermolecular Forces of Xenon Tetrafluoride
XeF4 is nonpolar. This means that it contains no permanently dipolar molecules; lacking a dipole. The only intermolecular forces that occur between nonpolar molecules are dispersion forces. If two XeF4 molecules were to bond together, the only intermolecular force that would occur would be the London Dispersion Force. This is when a molecule has momentary poles, temporary dipoles, and the electrons move quickly around the molecule. These electrons may be attracted to one side.
Structure of Xenon Tetrafluoride
XeF4 has a lewis strucutre that looks like this:
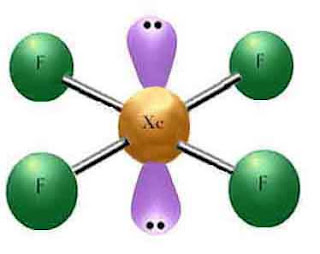
As you can see, there are six electron pairs around the xenon atom; four being covlent bonds to fluorine. The other two are unshared atom pairs. This molecule has a species type of AX4E2. This means that it is a square planar. Also, the bond angles will be at 90 degrees.
As you can see, there are six electron pairs around the xenon atom; four being covlent bonds to fluorine. The other two are unshared atom pairs. This molecule has a species type of AX4E2. This means that it is a square planar. Also, the bond angles will be at 90 degrees.
About Xenon Tetrafluoride
- Being a colorless crystal, Xenon Tetrafluoride was discovered in 1963 when this reaction occured:
- Xe + 2 F2 → XeF4.
- Because it is stable at room tempurature, Xenon Tetrafluoride is a lab curiosity. It can draw water out of air.
- This makes it useful because it can be used in humidifiers. Although it doesn't have many uses,
- it is exergonic meaning it releases energy. It is used as a decomposition agent of silicone rubber for
- analysing trace metal impurities in the rubber.
Subscribe to:
Comments (Atom)